Kybella Mountain View
While Kybella is an excellent product, it has been superseded in our practice by MyEllevate non-incision suture suspension necklift with Precision laser-assisted neck liposuction and skin tightening.
Kybella is one of the best options for getting rid of a double chin and excess fat around the jawline and chin. At An Enhanced You Cosmetic Surgery, we offer Kybella treatments in Mountain View for men and women looking for a non-surgical option for refining the appearance of their chin.
What Is Kybella
Kybella is an injectable product that reduces submental fullness without surgery. It is indicated for moderate to severe submental fullness due to subcutaneous fat. It can reduce fullness by one or two grades, meaning from severe to mild or moderate, or from moderate to mild. Skin quality should be good as injecting into an area with loose or excessively wrinkled skin can make the appearance worse.
Since submental fullness is a leading concern amongst all the things that bother people about their appearance, Kybella represents an advance in non-surgical treatment for this condition.
What to Expect
This is an office procedure. The area under the chin that has the unwanted fullness is outlined and a pattern of dots from a template is applied to ensure proper spacing of the injections. Depending on the surface area, about 20 small injections are made into the mid-layer of the fat, spaced about 1 cm apart. Side effects that occur most frequently are swelling and bruising. These are usually of short duration.
Kybella Recovery and Results
Swelling and some bruising around the chin and neck are common side effects. These issues will subside over time. Post-treatment instructions will be provided following your Kybella treatment in Mountain View.
The effect of Kybella is to break down the cell walls of the fat cells and thus destroy those cells. They will not grow back, so the effect is permanent. The fat is gradually absorbed over the next month or two, and repeat treatments are given at not less than one-month intervals.
Patients see a gradual decrease in submental fullness. The number of treatments varies between one and six, depending on the amount of fat to be treated and individual response.
Good Candidates for Kybella
If there is significant laxity of the lower face, jowl, and neck, and banding of the platysma muscle, then a lower face and neck lift would be appropriate.
Patient selection is important because if there are strong and visible platysma bands (detectable with grimacing) or loose skin, the removal of submental fat alone could worsen the appearance.
The best way to determine if Kybella is right for you is to come in for a consultation so that Dr. Lowen can properly examine your face and understand what your goals are for your treatment.
Alternatives to Kybella
Alternatives to Kybella that we offer include Precision Tx, a laser-assisted liposuction procedure that tightens the skin as well as removes fat. It is similar to Smartlipo but adapted to the lower face and neck with a smaller laser fiber and cannula.
The Cost of Kybella
The cost of your Kybella treatment in Mountain View will be discussed when you come in for your consultation. The total number of treatments needed will go into determining the overall price. Our team can also answer questions you have regarding the payment options available to you.
Schedule a Consultation
If you want to get rid of your double chin so you can feel more confident about your appearance, contact An Enhanced You Cosmetic Surgery today to schedule a consultation for your Kybella treatment in Mountain View.
Here are FAQ’s from Allergan, the maker of Kybella.
-
What is submental fullness?
Submental fullness, sometimes referred to as “double chin,” is a common, yet undertreated facial aesthetic condition. It can detract from an otherwise balanced and harmonious facial appearance – leading to an older and heavier look.
Submental fullness can impact a broad range of adults, including both women and men, and can be caused by aging, genetics and weight gain.
According to a 2015 survey by the American Society for Dermatologic Surgery (ASDS), over 2/3 of consumers are bothered by submental fullness – nearly as many as those bothered by lines and wrinkles around the eyes.
-
What is KYBELLA® (deoxycholic acid) injection?
KYBELLA® (deoxycholic acid) injection is the first and only FDA-approved injectable drug that contours and improves the appearance of submental fullness due to submental fat.
KYBELLA® is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
-
How does KYBELLA® work?
KYBELLA® is a non-human and non-animal formulation of deoxycholic acid, a naturally-occurring molecule in the body that aids in the breakdown and absorption of dietary fat.
When injected into subcutaneous fat, KYBELLA® causes the destruction of fat cells. Once destroyed, those cells cannot store or accumulate fat. After the aesthetic response is achieved, retreatment with KYBELLA® is not expected.
To avoid potential tissue damage, KYBELLA® should not be injected into or in close proximity (1-1.5 cm) to the marginal mandibular nerve, salivary glands, lymph nodes and muscles.
-
What are the results of KYBELLA®?
68.2% of subjects treated with KYBELLA® experienced a ≥1-grade improvement with KYBELLA® compared to 20.5% of placebo-treated subjects, based upon validated physician and patient measurements.
16% of patients experienced a ≥2-grade improvement with KYBELLA®, compared to 2% of patients who responded to placebo, based on validated physician and patient measurements
KYBELLA® treatment resulted in high patient satisfaction – 79% of KYBELLA®-treated patients reported satisfaction with their appearance in association with their face and chin.
Patients also reported improvement in the visual and emotional impact of submental fat when asked how happy, bothered, self-conscious, embarrassed, old and overweight they felt following treatment in relation to the amount of their submental fat.
Marginal mandibular nerve (MMN) injury occurred in 4% and dysphagia occurred in 2% of subjects. To avoid potential tissue damage, KYBELLA® should not be injected into or in close proximity (1-1.5 cm) to the MMN, salivary glands, lymph nodes and muscles.
The most common adverse reactions were edema/swelling, hematoma/bruising, pain, numbness, erythema and induration.
-
What are the side effects with KYBELLA®?
The safety profile of KYBELLA® is well-characterized.
KYBELLA® has been the focus of a global clinical development program involving over 20 clinical studies with more than 2,600 patients worldwide, of which over 1,600 have been treated with KYBELLA®.
The most common adverse reactions were edema/swelling, hematoma/bruising, pain, numbness, erythema and induration.
KYBELLA® is manufactured through a highly-controlled, FDA-regulated process and approved facility to ensure patient safety.
For more information, please see Important Safety Information
Kybella Before & Afters
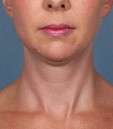
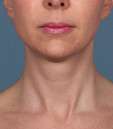
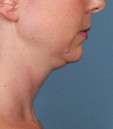
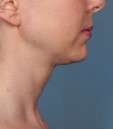
Photos courtesy of Allergan, Inc.
Unretouched photos of clinical trial patient taken before and after treatment with KYBELLA®.
Sex: F
Age: 35
BMI (before): 20 kg/m2
BMI (after): 19.6 kg/m2
Individual results may vary.
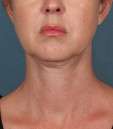
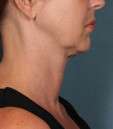
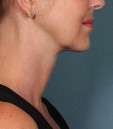
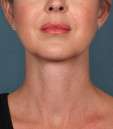
Photos courtesy of Allergan, Inc.
Unretouched photos of clinical trial patient taken before and after treatment with KYBELLA®.
Sex: F
Age: 39
BMI (before): 19.7 kg/m2
BMI (after): 20.4 kg/m2
Individual results may vary.

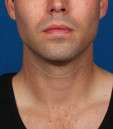
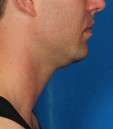
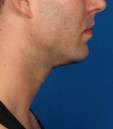
Photos courtesy of Allergan, Inc.
Unretouched photos of clinical trial patient taken before and after treatment with KYBELLA®.
Sex: M
Age: 34
BMI (before): 24.4 kg/m2
BMI (after): 25.8 kg/m2
Individual results may vary.
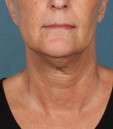
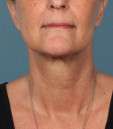
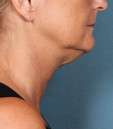
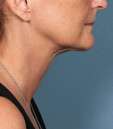
Photos courtesy of Allergan, Inc.
Unretouched photos of clinical trial patient taken before and after treatment with KYBELLA®.
Sex: F
Age: 55
BMI (before): 21.8 kg/m2
BMI (after): 21.8 kg/m2
Individual results may vary.

